Funded by NIH STTR/SBIR Program
- Awarded the NIH Small Business Technology Transfer (STTR) Phase 1 grant in 2017.
- Awarded the NIH SBIR Phase 2 grant in 2020.
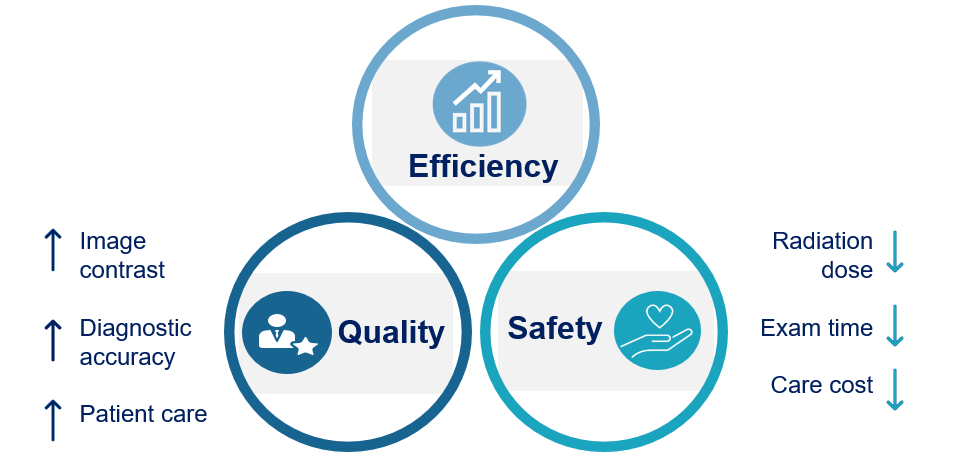

Improve safety and reduce the use of radiation/ contrast agent

Improve diagnostic accuracy and efficiency for treatment planning

Reduce cost and improve healthcare service provision and quality
Increase regulatory compliance, improve function and competitiveness of equipment to drive sales

Dr. Wang is a world leading expert in medical imaging with over 20 years of experience. Dr. Wang also maintains longstanding R&D collaborations with large OEMs including Philips, Siemens, Samsung and Neusoft.

Mr. Liu has more than 20 years of experience in international business development, marketing, investment, M&A and strategy in the healthcare industry.

Mr. Korczykowski served as a Senior Advisor at Brookline Capital Markets in NY and Senior Director, Clinical Operations at a publicly traded Biotech Company. He has 15 years of experience advancing clinical diagnostics and drug discovery & development efforts.

10 years of Experience in Medical Imaging Software R&D

Adjunct Professor of UCLA David Geffen School of Medicine

10 years Medical Software Development

Associate Professor of Computer Sciences, Pepperdine University

8 years Medical Software Development

Director of the UCLA Stroke Center
Professor of Neurology
UCLA Department of Neurology
Director, UCLA Stroke Center
Director, Neurovascular Imaging Research Core

Clinical Professor, Department of Radiation Oncology. Clinical Professor and Director, Division of Radiation Physics, City of Hope

Associate Professor of Radiologic Science at University of Pennsylvania

Director at Center for Functional NeuroImaging. Professor of Neurology & Radiology at University of Pennsylvania

Member of Science Advisory Board
Professor of Radiology; Director of Physics & Biology in Medicine Graduate Program; UCLA David Geffen School of Medicine

Associate Professor of Radiology Director of MRI Washington School of Medicine in St Louis
